Our Commitment to Excellence
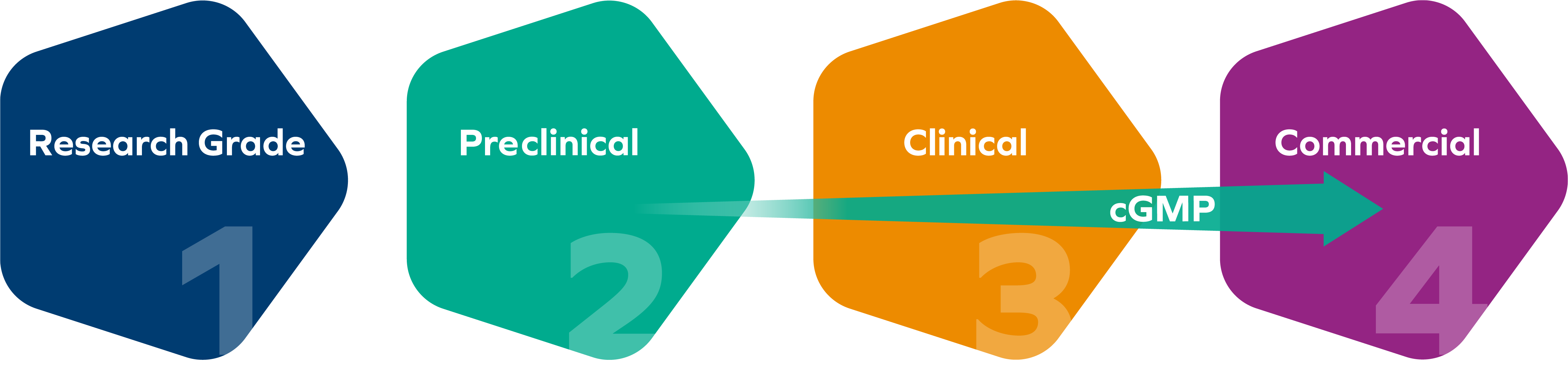
Strict adherence to the highest quality standards defines who we are, whether manufacturing nucleic acids for R&D or commercially manufacturing and analyzing nucleic acid active pharmaceutical ingredients (APIs) and drug products. We operate according to the current Good Manufacturing Practices (cGMP) as regulated by the German authorities, Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the Japanese Pharmaceutical and Medical Devices Agency (PMDA). No matter where you are at in the drug development process, we work alongside our clients to achieve our shared vision of a safer and healthier world and deliver the quality you require.
Covering the Entire Drug Development Lifecycle