Nucleic Acid Manufacturing and Analysis
BioSpring is an industry leader in nucleic acid manufacturing technology.
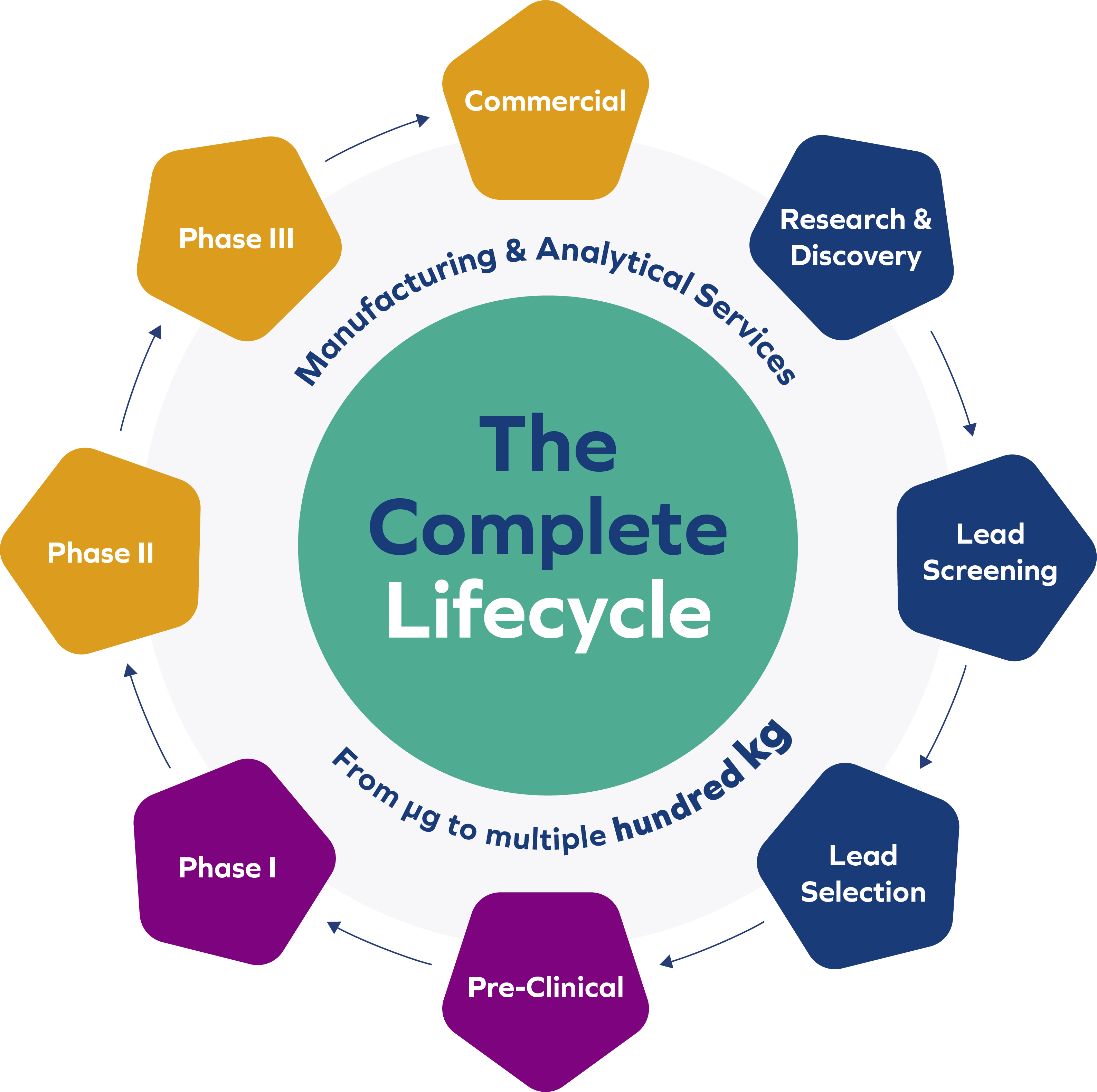
As an expert in this field with a quarter century of experience, BioSpring successfully delivers innovative solutions for manufacturing and analysis of nucleic acid therapeutics and diagnostics spanning the complete lifecycle: from discovery to commercial use. Clients can rely on BioSpring’s precision and strict cGMP compliance – guided by our expertise, passion for innovation and more than 25 years of experience.
Our aim is a highly collaborative partnership to unleash the full potential of our clients' technology - making great strides and breakthroughs possible.
Learn more about our nucleic acid servicesAt Any Scale
We support our clients from early small-/mid-scale manufacture of their drug development candidates to late-stage clinical and commercial manufacturing, including all related analytical activities.
- cGMP manufacturing for clinical Phase I – III: Multiple-hundred kg annual capacity
- Process validation and commercial manufacture
- Manufacturing of oligonucleotides for R&D, as well as lead candidate screening and development
- Process development – more than 140 successful programs each year
- Scale-up, scale-down and process transfer
- Pre-clinical non-GMP batch manufacture
- Regulatory tox batch manufacture
- Generation of reference standards